Table of Contents
PDL1 in one nutshell
What is PDL1?
Programmed death-ligand 1 is a protein that in humans is encoded by the CD274 gene. It is a transmembrane protein that suppresses the cellular immunity during particular events such as physiological event like pregnancy, physiopathological event like protecting normal cells in context of infection/inflammation and to prevent autoimmune disease. Cellular immunity is most effective against cells infected with viruses, intracellular bacteria, fungi and protozoans. Malignant cells also take advantage of this protective mechanism to escape immunne surveillance. The 2015 revolution in immunotherapy is based on preventing cancer to escape immune surveillance by inhibiting interaction between PD1 (expressed by CD8) and PDL1 (expressed by tumoural cells) using an engineered humanized IgG4 antibody targeting either PD1 or PDL1.
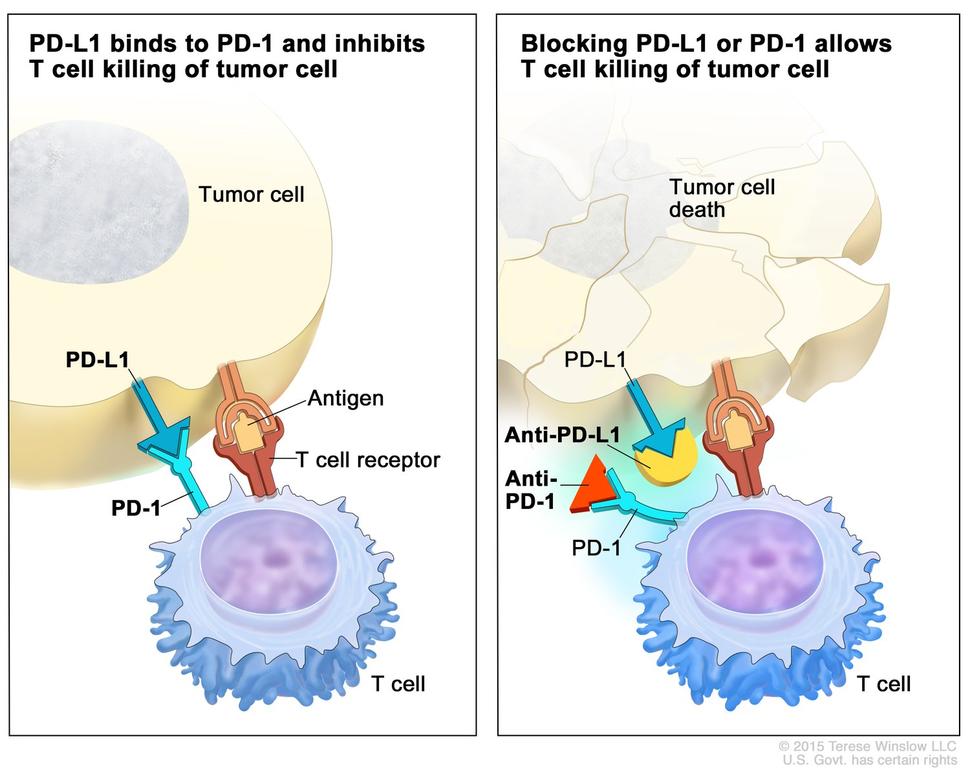 In the upper right picture, two humanized antibodies IgG4 anti-PD1 and anti-PDL1 are respectively represented by a red triangle and an incomplete yellow circle
In the upper right picture, two humanized antibodies IgG4 anti-PD1 and anti-PDL1 are respectively represented by a red triangle and an incomplete yellow circle
Can I use any PDL1 for any type of tumour?
No. There is no agnostic PDL1 assessment and predicting response to therapy relies on clinical trials which developed a specific PDL1 test for a specific drug and a specific cancer summarized as the 3D concept below.
The 3D PDL1 concept
Cancer immunotherapy development rely on clinical trial run by pharmaceutical companies. The major players are Merck, Bristol Myers Squibb (BMS), Genentech and AstraZeneca. The first “D” of the 3D concept identifies specific Drug developed by these companies which are respectively Pembrolizumab, Nivolumab, Atezolizumab and Durvalumab. The second “D” identifies the disease and finally the third “D” identifies the Diagnostic test. Unfortunately, these pharmaceutical companies have chosen to utilize different proprietary PDL1 IHC assays involving different clones and different reagents. Two "Blue Print" studies have shown relative good correlation between these assays to detect tumoral cells but poor agreement for assessment of PD-L1 staining on immune cells making the assays not readily interchangeable given the trend for CPS assessment over TPS. Indeed close to critical threshold of positivity, the different analytical sensitivity of these kits provide different results. Therefore IHC lab are forced to utilize these different proprietary IHC assays. It is possible to match a given proprietary IHC assay by calibrating a “Lab Developed Test” (LDT) to achieve the same sensitivity/specificity of a given IHC assay. This was realized across Canada (from 2018-2020) to match the analytical sensitivity of the Dako PD-L1 IHC 22C3 pharmDx kit using different platforms (Canadian Multicenter Project on Standardization of Programmed Death-Ligand 1 Immunohistochemistry 22C3 Laboratory-Developed Tests for Pembrolizumab Therapy in NSCLC).
PDL1 test interpretation
The three major predictors of response to immunotherapy are the tumoural PDL1 expression, the number of lymphocytes within the tumoural bed (TILs) and the mutational load. The first assessment of response to therapy was only looking at the tumoural expression corresponding to the proportion of PD-L1 positive tumoural cells: Tumoural Proportion Score (TPS). Later researchers realized that only looking at TPS was not powerful enough to predict response to therapy (especially for other type of tumours). Since most TILs and macrophages involved in the tumoral bed are PDL1 positive, a new scoring system Combined Proportion Score (CPS) gathered two predictors that are tumoural PDL1 expression and immune cells (Lymphocytes and macrophages) within the tumoural bed.
The currently available 3D PDL1 applications in Alberta
(Last update: April 4, 2023)
| Disease | Drug | Diagnostic Test | Method | Thresholds | Report template with PubMed ref |
|---|---|---|---|---|---|
| Breast - Triple Negative | Pembrolizumab (Merck) | Dako PD-L1 IHC 22C3 pharmDx | CPS | Negative < 10, Positive >= 10 | TNBC |
| H&N - SCC | Pembrolizumab (Merck) | Dako PD-L1 IHC 22C3 pharmDx | CPS | Negative < 1, Positive >= 1 and < 20, Positive >= 201) | H&N |
| Lung - NSCLC | Pembrolizumab (Merck) | Dako PD-L1 IHC 22C3 pharmDx | TPS | Negative <1%, Low positive >=1% and <50%, positive >=50% | NSCLC |
| Uterine Cervix - SCC and adenocarcinoma | Pembrolizumab (Merck) | Dako PD-L1 IHC 22C3 pharmDx | CPS | Negative < 1, Positive >= 1 | Cervical Cancer |
| Upper GI (esophageal and gastric) - adenocarcinoma | Nivolumab (BMS) | Dako PD-L1 IHC 28-8 pharmDx | CPS | Negative < 5, Positive >= 52) | Upper GI adeno |
Currently FDA approved 3D applications in Metastatic/ Unresectable Malignancies
| Drug | Disease Indication | Phase of Study (ClinicalTrials.gov Identifier) | Response Rate [95% CI] | Median Overall Survival (mon) |
|---|---|---|---|---|
| Nivolumab | First-line melanoma | Phase III NCT01844505 | 45% [39.1%–50.3%] | 36.9 |
| Pembrolizumab | First-line melanoma | Phase III NCT01866319 | 42% [38.1%–46.5%] | 32.7 |
| Nivolumab | Second-line NSCLC | Phase III (squamous) NCT01642004 Phase III (non-squamous) NCT01673867 | 20% [14%–28%] (squamous) and 19% [15%–24%] (non-squamous) | 9.2 (squamous) and 12.2 (non-squamous) |
| Pembrolizumab | First-line NSCLC (PD-L1 ≥ 50%) | Phase III NCT02142738 | 44.8% [36.8%–53%] | 30 |
| Nivolumab | Third-line SCLC | Phase I/II NCT01928394 | 12% [6.5%–19.5%] | 4.4 |
| Nivolumab | Second-line RCC | Phase III NCT01668784 | 25% [not mentioned] | 25 |
| Nivolumab | Second-line UCC | Phase II NCT02387996 | 19.6% [15.0%–24.9%] | 8.74 |
| Pembrolizumab | Second-line UCC | Phase III NCT02256436 | 21.2% [16.4%–26.5%] | 10.3 |
| Pembrolizumab | First-line Merkel cell carcinoma | Phase II NCT02267603 | 56% [41.3%–70%] | NR |
| Pembrolizumab | Any refractory MSI-H tumor | Phase II NCT01876511 | 53% [42%–64%] | NR |
| Cemiplimab | Cutaneous SCC not amenable to local resection | Phase I NCT02383212 and NCT02760498 | 47% [34%–61%] | NR |
| Nivolumab | Later-line MSI-H CRC | Phase II NCT02060188 | 31.1% [20.8%–42.9%] | NR |
| Pembrolizumab | Later-line MSI-H | CRC Phase II NCT02460198 | 33% [21%–46%] | 31.4 |
| Pembrolizumab | Third-line (PD-L1 ≥ 1%) gastric and gej adenocarcinoma | Phase II NCT02335411 | 22.7% [13.8%–33.8%] | NA |
| Nivolumab | HCC | Phase I/II NCT01658878 | 20% [15%–26%] | NR |
| Nivolumab | Second-line head and neck SCC | Phase III NCT02105636 | 13.3% [9.3%–18.3%] | 7. 5 |
| Pembrolizumab | Second-line head and neck SCC | Phase III NCT02252042 | 14.6% [10.4%–19.6%] | 8.4 |
| Pembrolizumab | First-line head and neck SCC (PD-L1 ≥ 1%) | Phase III NCT02358031 | 19% [not mentioned] | 12.3 |
| Nivolumab | Relapsed/refractory cHL | Phase II NCT02181738 | 69% [63%–75%] | NR |
| Pembrolizumab | Relapsed/refractory cHL | Phase Ib NCT01953692 | 65% [48%–79%] | NR |
| Pembrolizumab | Relapsed/refractory PMBCL | Phase II NCT02576990 | 45% [32%–60%] | NR |
| Pembrolizumab | Second-line (PD-L1 ≥ 1%) cervical carcinoma | Phase II NCT02628067 | 14.6% [7.8%–24.1%] | 11 |
Requirements to validate a PDL1 test
- The pre-analytical requirements which are are validated by the manufacturer. These requirements are generally similar to other class II IHC assays (for instance Breast Biomarkers (ER, PR and HER2)
- Possible analytical issues
- The type of tissue which is related to the performed PDL1 readout and namely the TPS and the CPS readout methods.
- The minimum amount of tissue to perform the test: a number of 100 tumoural cells has been established by the first successful MERCK clinical trial regarding the 3D - Pembrolizumab/NSCLC/22-C3 PDL1 assay. Since then, the same number is required for other applications. An Albertan study has however demonstrated the potential of false results (and especially false negative results) with such tiny amount of malignant cells. This is related to the spatially and temporally heterogeneous nature of PDL1 expression in solid tumours.
PDL1 workflow in Alberta
- Primary lung NSCLC PDL1 ordered reflexively by the signing pathologist
- For all other specimens, PDL1 can be ordered by oncologists only!
- Pathologists
- ensure the request is appropriate and fulfills the inclusion criteria for testing.
- select the most appropriate block with adequate tumour cell number (minimum 100)
- Prefer tissue for all requests (especially for CPS assessments)
- Cytology and EBUS acceptable for Lung (TPS assessment)
- Selecting the best block at the time of sign out would take 1 minute but might save one day of TAT for Biomarker reporting
- request 5x unstained slides from their lab
- slides sent to Edmonton for staining
- slides returned to Calgary for reporting by the Biomarker Group
- Typical turnaround: 5-10 days, longer if case from outside Calgary
TRK workflow in Alberta
- Tumours with high prevalence of NTRK fusions (i.e. secretory carcinoma, infantile fibrosarcoma, congenital mesoblastic nephroma) → order NGS directly
- Tumours with low prevalence of NTRK fusions (i.e. all others) → NTRK IHC as screen
- Can be ordered by oncologist only! (requests go to: FMC consult desk (Calgary) and to the EZIHC Lab (Edmonton)
- Pathologists cannot request this test but are responsible for ensuring the request is appropriate (for example no cytology samples, no decal samples, tumour is present in the block, etc)
- 4x unstained samples are sent to Edmonton for staining and returned to Calgary for reporting by the Biomarker Group
